
Contact Details:
Instron
Coronation Road
High Wycombe
Bucks
HP12 3SY
United Kingdom
Tel: +44 1494 456815
Alt. Tel: +44 1494 464646
Fax: +44 1494 456814
Send Enquiry | Company Information
LIGAGEN
Company News Monday, March 17, 2014: Instron
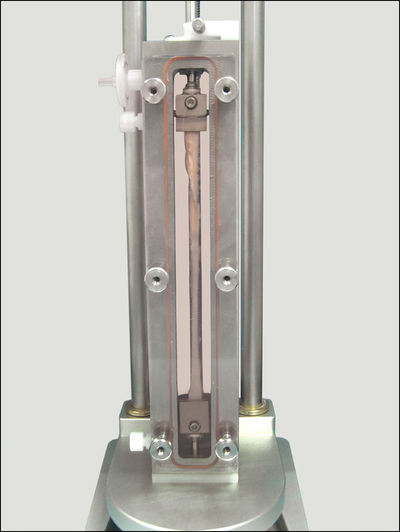
Instron, a leading provider of testing equipment designed to characterise native tissue, stimulate live cell culture, and evaluate mechanical properties of materials and components, offers the LigaGen system. The LigaGen architecture provides a physiologic support system that enhances metabolic conditions for cell growth and maintenance in a 3D environment. Physiologic parameters are feedback-controlled for culture reproducibility.
The LigaGen’s flexible hardware and computer control systems allow for the development of a wide variety of automated experimental protocols with varying levels of complexity (frequency, force magnitude and application profile) and can accommodate scaffolds up to 150 mm in length.
Tissue engineering and regenerative medicine is dedicated to creating new tissue engineered medical devices that replace an/or enhance tissue function that has been impaired by disease, injury, or age. Research has shown that stimulating cells and tissues during development in vitro, results in tissue more similar to native tissue because tissues are normally exposed to a variety of biomechanical signals in vivo.
Clinical Example
The current choices for replacement tendon or ligament grafts include autologous grafts from other parts of the patient or allograft cadaver tendons or ligaments. Autologous procedures are associated with long recovery times and significant harvest site pain, while allogenic grafts risk both disease transmission and infection. Tissue engineering provides an attractive alternative, providing living replacement graft made with the patients own cells. Culturing functional tissue engineered grafts requires bioreactors capable of delivering controlled stresses/strains to cell-seeded scaffolds at specified frequencies inside a nutrient rich environment.
Mutilating injuries of the hand – due to trauma or disease – sometimes requires reconstruction, and often times, there is not enough autologous tendon to complete the surgery. Stanford University Medical Center is developing a tissue engineered alternative. Their 2011 publication in Tissue
Engineering Part A concluded that material properties of re-cellularized human hand tendon were enhanced by dynamic-conditioning in Instron’s LigaGen bioreactor.
Cited Source: Colin Y.L. Woon, Armin Kraus, Shyam S. Raghavan, Brian C. Pridgen, Kai Megerle, Hung Pham, and James Chang. Tissue Engineering Part A. October 2011, 17(19-20): 2561-2572. doi:10.1089/ten.tea.2010.0701.
About Instron
Instron is a leading provider of test equipment for the material and structural testing markets. A global company providing single-source convenience, Instron manufactures and services products used to test the mechanical properties and performances of various materials, components and structures in a wide array of environments.
Instron systems evaluate materials ranging from the most fragile filament to advanced high-strength alloys, providing customers with comprehensive solutions for all their research, quality and service-life testing requirements. Additionally, Instron offers a broad range of service capabilities, including assistance with laboratory management, calibration expertise and customer training.
